President Donald Trump’s choice to lead the Food and Drug Administration (FDA) flipped a question about vaccine processes around onto a top Democratic senator during his confirmation hearing on Thursday, advising them to ask former President Joe Biden why he skipped a key step when it came to the COVID-19 booster.
Dr. Marty Makary, Johns Hopkins School of Medicine professor and former Fox News medical contributor, went before the Senate Committee on Health, Education, Labor and Pensions (HELP), during which he answered questions regarding vaccines, chronic illness, food safety and abortion.
“So if you are confirmed, will you commit to immediately reschedule that FDA Vaccine Advisory Committee meeting to give the expert views?” Sen. Patty Murray, D-Wash., asked Trump’s FDA pick.
INSIDE ELON MUSK’S HUDDLE WITH GOP SENATORS: DOGE HEAD TOUTS $4M SAVINGS PER DAY
Dr. Marty Makary pointed out the Biden administration’s decision to skip key committee meetings when authorizing vaccines in response to a top Democrat’s question. (Reuters)
Her question came in reference to an FDA vaccine meeting that was reportedly postponed at the last minute.
“I would reevaluate which topics deserve a convening of the advisory committee members on [Vaccines and Related Biological Products Advisory Committee] and which may not require a convening,” Makary replied, noting he was not a part of the decision.
Asked again by Murray, the FDA commissioner nominee said, “Well, you can ask the Biden administration that chose not to convene the committee meeting for the COVID vaccine booster.”
EXCLUSIVE: ELON MUSK PAC THANKS TRUMP FOR ‘SAVING THE AMERICAN DREAM’ IN NEW MILLION-DOLLAR AD
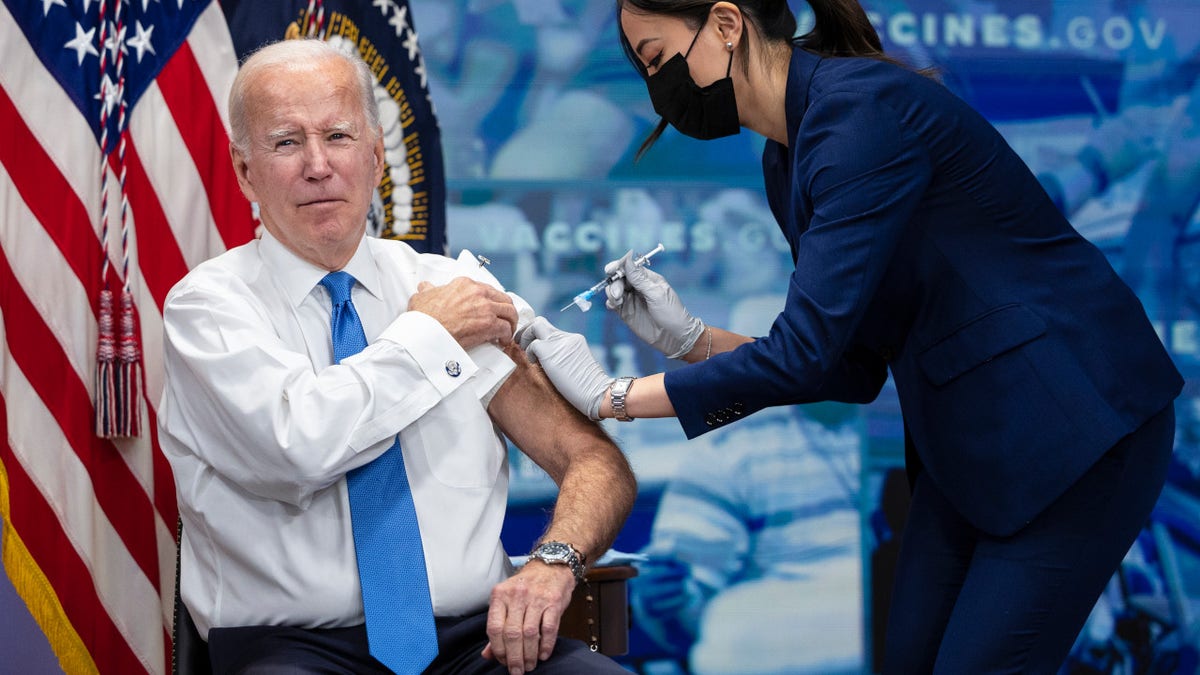
President Joe Biden receives a COVID-19 booster shot inside the South Court Auditorium at the White House on Oct. 25, 2022. (Tom Brenner for The Washington Post via Getty Images)
In 2021, Biden’s administration notably pushed through FDA approval for a COVID-19 booster for everyone over the age of 18. Per a press release at the time, “The FDA did not hold a meeting of the Vaccines and Related Biological Products Advisory Committee on these actions as the agency previously convened the committee for extensive discussions regarding the use of booster doses of COVID-19 vaccines and, after review of both Pfizer’s and Moderna’s EUA requests, the FDA concluded that the requests do not raise questions that would benefit from additional discussion by committee members.”
At the time, committee member Dr. Paul Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia remarked, “We’re being asked to approve this as a three-dose vaccine for people 16 years of age and older, without any clear evidence if the third dose for a younger person when compared to an elderly person is of value.”
HOUSE GOPERS HOPE TRUMP KEEPS DOOR TO MINERAL DEAL OPEN FOR UKRAINE DESPITE OVAL OFFICE DISPUTE

The Food and Drug Administration headquarters in White Oak, Maryland, on Aug. 29, 2020. (Reuters/Andrew Kelly/File Photo)
Fox News Digital asked Murray whether she was similarly concerned by Biden’s decision. The senator said in a statement, “In 2022, I had confidence that our public health agencies were following the latest science and listening to public health experts. I do not have that confidence now.”
“We’re talking about Trump and RFK Jr. canceling a routine meeting that has taken place annually, for at least 30 years, to make recommendations for which influenza strains should be included in the flu vaccines for the upcoming flu season – there has been zero justification for its cancellation or any information about when it would be rescheduled,” she continued. “The flu vaccine is safe, effective, and lifesaving – we need this advisory committee to meet so manufacturers have enough time to prepare the correct vaccines.”
Ahead of the Thursday hearing, Murray and fellow HELP Democratic Sens. Tammy Baldwin of Wisconsin and Angela Alsobrooks of Maryland penned a letter to Makary, telling him, “We intend to use your nomination hearing next week to understand whether you support this ill-informed measure to slow critical public health decision-making.”
GOP REBELS FIRE WARNING SHOT IN SHUTDOWN SHOWDOWN: NO DOGE, NO DEAL

Cassidy is chairman of the HELP committee. (Ting Shen/Bloomberg via Getty Images)
HELP Chairman Bill Cassidy, R-La., also inquired about the postponed meeting, asking Makary, “How will you ensure that advisory committees remain objective, transparent and still benefiting from the necessary expertise of external experts?”
CLICK HERE TO GET THE FOX NEWS APP
The nominee told Cassidy, “You have my commitment to review what the committees are doing [and] how they’re being used.”
“As you know, I was critical when that committee was not convened at all during one of the COVID booster guidance decisions by the FDA,” Makary noted.
He recalled that FDA leadership “at the time argued that they’re advisory, and we don’t have to convene them. That was repeatedly, throughout the Biden administration.”


