‘Blink and you’ll miss it’ is an understatement in the world of protein biology. A blink lasts hundreds of milliseconds. Proteins operate on a much shorter timescale — on the order of a millisecond, or even faster. “To capture many of the domain motions of proteins that are linked to function, you would have to be able to go down to a microsecond timescale,” says Ulrich Lorenz, a chemist at the Swiss Federal Institute of Technology in Lausanne.
Working on that timescale has long stymied biologists, but the rapid evolution of time-resolved cryo-electron microscopy (TR cryo-EM) over the past several years has made it possible to reconstruct dynamic processes with near-atomic detail.
How protein-slayer drugs could beat some of the cruellest cancers
Beyond fundamental biological insights, a deeper understanding of protein dynamics could guide the development of more effective medicines and boost protein-engineering efforts. “Time-resolved cryo-EM could be the natural choice — or only option — to capture [protein] conformations that could be potentially better targeted by drugs,” says Youdong Mao, a physicist at Peking University in Beijing, China.
But the technique is niche: these methods require considerable expertise and specialized equipment, and researchers are still working out how to interpret the resulting data. “Nature is under no obligation to be simple,” cautions Radoslav Enchev, a biochemist at the Francis Crick Institute in London. And until more laboratories get the tools needed to perform TR cryo-EM, progress in this exciting field will continue to be sporadic.
From stills to cinematography
The basics of cryo-EM are straightforward: researchers apply protein solutions to a ‘grid’ substrate and flash-freeze them into a glassy structure known as vitreous ice. Using a transmission electron microscope, they then image individual molecules in many orientations — imagine chunks of fruit embedded in jelly. Given enough examples, these 2D ‘projections’ can be reconstructed into a high-resolution 3D structure.
Skilled users can produce structures sharp enough to resolve individual hydrogen atoms. But most experiments image just a single, relatively stable conformation. Structural biologist Stephen Muench at the University of Leeds, UK, struggled for years with the limitations of his cryo-EM studies of a protein known as vacuolar ATPase, a molecular motor that spins as it pumps protons across lipid membranes. “There were a lot of questions we just couldn’t answer with static structures,” says Muench.
More generally, says Lorenz, biologists have had to choose whether to achieve high resolution in time or in space. Live-cell ‘super-resolution’ experiments can visualize single proteins at millisecond timescales but cannot discern detailed structural features. Nuclear magnetic resonance can record relatively fast transitions between protein conformations with high resolution, but is limited to small targets. And although time-resolved X-ray crystallography can distinguish both atomic detail and incredibly rapid, femtosecond-scale movement, it does so in a highly constrained setting. “Protein dynamics don’t naturally occur in a crystal,” explains Lorenz.
These ‘movies’ of proteins in action are revealing the hidden biology of cells
In principle, cryo-EM provides an excellent fit for time-resolved experiments. Given enough particles, researchers could theoretically reconstruct every conformational state that a protein can assume. The trick is to get these dynamic processes to unfold in lockstep, such that the 3D reconstructions can be assembled into a molecular movie. This can be achieved by taking a sample of protein at equilibrium, introducing a ligand or other molecular trigger that initiates movement, and then rapidly freezing the sample at various times after the reaction starts.
In 1995, for instance, Nigel Unwin, a structural biologist at the MRC Laboratory of Molecular Biology in Cambridge, UK, prepared a grid with the receptor for the neurotransmitter acetylcholine and sprayed it with a solution containing acetylcholine1. By quickly freezing the mixture, Unwin captured changes that occurred in the receptor within five milliseconds of acetylcholine binding. Cryo-EM spatial resolution was poor in those days, but other groups have subsequently leveraged similar ‘spray-and-mix’ approaches to produce detailed dynamic reconstructions with modern, high-resolution instruments.
However, spray-and-mix methods can also introduce considerable uncertainty owing to inefficient mixing between protein and ligand. “Diffusion is happening, so nothing is really under control,” says Joachim Frank, a biophysicist at Columbia University in New York City, who shared the 2017 Nobel Prize in Chemistry for his pivotal role in advancing cryo-EM for structural biology. “We wanted to develop a method in which we have control of everything.”
As an alternative, Frank’s group began working on polymer-based microfluidic chips containing tiny channels, within which a protein and its ligand can be precisely mixed before being sprayed onto a grid and frozen. They described their first-generation instrument in 20152, and several other groups have subsequently devised alternative ‘mix-and-spray’ systems.
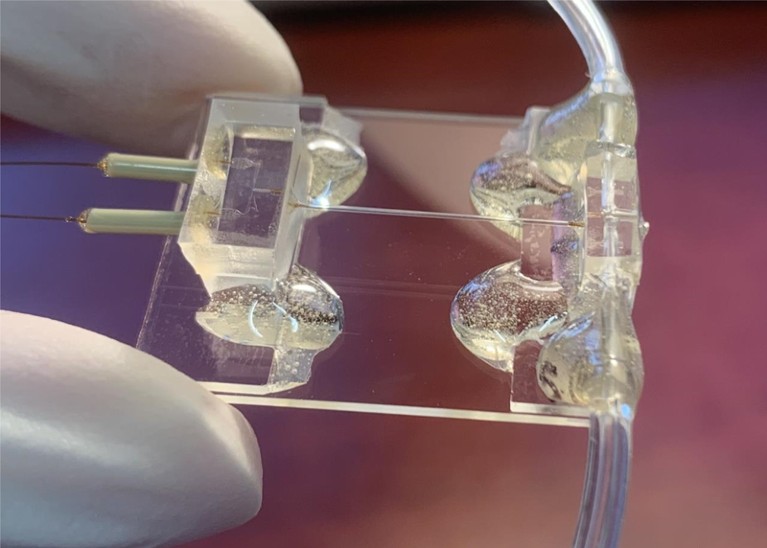
A microfluidic chip comprising three parts: a micromixer, a microreactor and a microsprayer.Credit: S. Bhattacharjee et al./Cell
Contemporary chips incorporate precisely engineered, zigzagging channels for rapid, efficient sample mixing and smooth fluid flow, and mix-and-spray has become the method of choice for many TR cryo-EM practitioners. Last year, for example, Frank and his colleagues used their latest-generation microfluidic design to study how ribosomes are recycled after protein synthesis to begin translation of a new polypeptide chain3. “We can see how the ribosome is opened up like a clam-shell upon the interaction with a particular molecule that pries the whole thing open,” says Frank. In April, Muench and his collaborators employed their own mix-and-spray system to study actomyosin complexes from muscle fibres, revealing crucial details about how the myosin protein stores and subsequently releases the energy that powers muscle contraction over the course of 120 milliseconds4.
Still, there’s plenty of room for improvement. For one thing, microfluidics-based sample preparation requires a lot of material. “In freezing one time point, you consume several microlitres of sample, which normally is all the protein you can purify over several months,” says Mao. His team pursued an alternative approach to study the protein-degrading proteasome complex, using reaction conditions that slowed proteasome activity to a crawl so that time-resolved data could be collected using more conventional cryo-EM methods.
An evolving landscape
Researchers led by Rouslan Efremov, a structural biologist at the VIB-VUB Center for Structural Biology in Brussels, have devised a mix-and-spray system that reduces sample-size requirements by an order of magnitude. Their microfluidic device mixes a protein and a ligand in tiny droplets, allowing them to rapidly react in a confined space before they are launched onto the grid using a laser-based mechanism5. “The main advantage is that this consumes only about 100 nanolitres per grid,” says Efremov, who has used this approach to study complexes that are responsible for protein folding and bacterial metabolism.
Faster frame rates are also in demand. Existing microfluidic approaches have a ‘speed limit’ of around 5 milliseconds, and although many conformational changes occur in this time frame, others do not. Furthermore, millisecond-scale resolution preferentially captures major intermediates and endpoints for a protein conformational change but misses shorter-lived transition states that are essential for understanding the events leading up to that change.
Catching proteins at play: the method revealing the cell’s inner mysteries
Lorenz’s group took a gamble in its bid to break the millisecond barrier6. First, the researchers freeze their protein sample on a grid in the presence of an inactive, chemically ‘caged’ ligand or activator. The frozen sample is then illuminated with a laser to release the activator compound, after which a second beam is used to quickly melt the ice. After an appropriate interval, the sample is rapidly refrozen, capturing conformational changes occurring in as few as 5 microseconds.
Remarkably, this melting and revitrification strategy does not seem to negatively affect proteins, and Lorenz notes that it can even improve the resolution of the 3D reconstruction by knocking the protein particles into a wider range of orientations. His team is currently using this method to study a variety of viruses and investigate how rapid conformational changes by viral proteins aid host-cell infection. And even faster snapshots might be in reach. “I think 10 nanoseconds or so should be easily possible,” says Lorenz, adding that such resolution could capture ultra-fast events, such as the folding of protein structural domains.
When it comes to TR cryo-EM, even minor details matter. For example, the ice layer on the grid needs to be thick enough to protect the proteins from air, which can damage molecules and render them uninterpretable, but also thin enough to maximize image contrast. In a preprint published in April, Enchev and his colleagues presented a platform called Chronobot to tackle this problem7. Here, samples are mixed in a reusable glass microfluidic chip and then sprayed onto the grid, which is imaged with a high-speed camera immediately before freezing. A deep-learning algorithm then assesses the ice quality in each sector of the grid while also optimizing future sample-preparation conditions on the basis of those results. This helps the team to avoid wasting valuable instrument time on bad samples. “This rapid iteration away from the expensive microscope allows us to make sure that we would get a high-resolution data set of any grid that passes this quality control,” says Enchev.